Mgcl2 Ionic or Covalent
ESCTmix term and CH-pi pi-pi interaction. Which is the correct formula including charge of a nitrate ion.
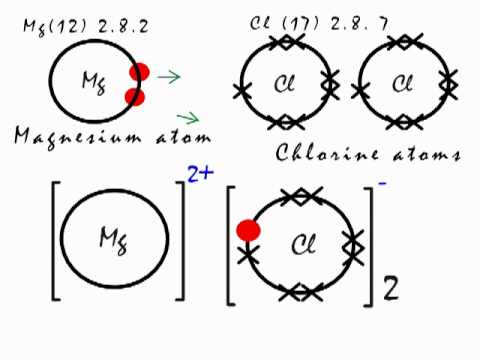
Ionic Bonding In Mgcl2 Magnesium Chloride Youtube
01 005 001.

. Alkanes are saturated organic compounds which are made up of C and H atoms. Our approach reveals the underlying chemistry that compliments the covalent. ES term steric hindrance.
Is a relative measure of an atoms attraction for electrons it shares in a covalent bond. Molecular structure does not easily identify the intricate noncovalent interactions that govern many areas of biology and chemistry including design of new materials and drugs. Ionization energy atomic and ionic radii electron affinity electro negativity and valency.
An effort made to mitigate the crisis and current circumstances forced by the major spread of the novel corona virus. C oxygen occupies more space than hydrogen. Alkanes have the general formula of CnH2n2.
D hydrogen is much more electronegative than oxygen. It takes 420 min for the concentration of a reactant in a first-order reaction to drop from 045 M to 032 M at 25C. The hydrogen atom needs one more electron and the oxygen atom needs two more electrons to complete the octet hence both of these will share the valence electrons with each other.
We develop an approach to detect noncovalent interactions in real space based on the electron density and its derivatives. Therefore the sharing of electrons between hydrogen and oxygen atom makes a covalent bond in H 2 O or water molecules. Synthesis of N-6-Bromohexyl phthalimide Typically 10 g of phthalimide 13594 mmol and 221 g 6783 mmol of caesium carbonate Cs 2 CO 3 was dissolved in 100 mL of anhydrous acetonitrile in a 250 mL long-necked round-bottom flask and the mixture was stirred at 60 C for 05 hThen 50 mL of 16-dibromohexane 32381 mmol was added and the mixture was further.
DOCKTITE is a fast and user friendly method for covalent. O 222 min O 860 min O 130 min O 284 min O 137 min. Vector of concentrations default.
The PIEDA components have correspondences such as ionic bond. The lone pair electrons can never take parts in chemical. Illustration of the significance of liquid crystal films doped with chiral ligand-capped Au nanoparticles forming microlens arrays in water when suspended in hollow grids.
Periodic law and the modern periodic table. S p d and f blocks. Illustration of the cholesteric liquid crystal microlens array with.
EX term hydrogen bond. The Ministry of Education and Higher Education MEHE the Center for Educational Research and Development CERD as well as public and private school administrations and teachers are all collaborating to provide an effective and impactful. Compared to ionic and covalent bonding Hydrogen bonding is a weaker form of chemical bonding.
B oxygen is much more electronegative than hydrogen. Other ions could be used. Magnesium chloride is the name for the chemical compound with the formula MgCl 2In addition to the anhydrous form MgCl 2 comes in its various hydrates MgCl 2 nH 2 OThese salts are typical ionic halides being highly soluble in waterThe magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great Salt.
This kind of chemical bonding existing between two unlike charged particles is known as an electrovalent bond. How long will it take for the reaction to be 90 complete. A it is an ionic bond.
Complete a net ionic equation for each proton-transfer reaction using curved arrows to show the flow of electron pairs in each reaction. For example MgCl2 the magnesium ion and chlorine ions are held together by force of electrostatic attraction. Alkane contains sp3 hybridized carbon atoms with four sigmas σ bonds every hydrogen atom is connect.
Such lenses are analogous to the structure of the compound eyes of insects and are sensitive to the polarization of the light.

Is Mgcl2 Ionic Or Covalent Nature Of Chemical Bond In Mgcl2

Is Mgcl2 Magnesium Chloride Ionic Or Covalent Youtube

Comments
Post a Comment